Abstract
Multiple myeloma is an incurable malignant hematopoietic blood cancer. It develops from genetic disposition and abnormal plasma cells with over 30,000 new cases diagnosed in the United States every year. The 5-year relative survival rate for myeloma patients if diagnosed at early stage is 79.5%, however majority of patients are diagnosed once the cancer has already metastasized which decreases the 5-year relative survival rate to only 59%. The standard care for treating newly diagnosed multiple myeloma (NDMM) is three-drug induction therapy followed by high-dose melphalan (HDM) and autologous stem cell transplantation (ASCT). While there is no cure for multiple myeloma, diagnosis and standard therapy leads to prolonged remission and improved quality of life. Identifying genetic predictors for positive treatment responses and survival represents an unmet medical need.
Long non-coding RNAs (lncRNAs) play important regulatory roles in promoting cancer growth and metastasis and due to their tissue-specificity they are becoming important players for development of personalized therapies. In addition, lncRNA-protein interactions have shown to be vital for biological processes associated with cancer, but currently there is a lack in our understanding of their role in initiation or progression of myeloma. Further, the roles of lncRNAs as biomarkers or targets for myeloma therapies have not yet been defined.
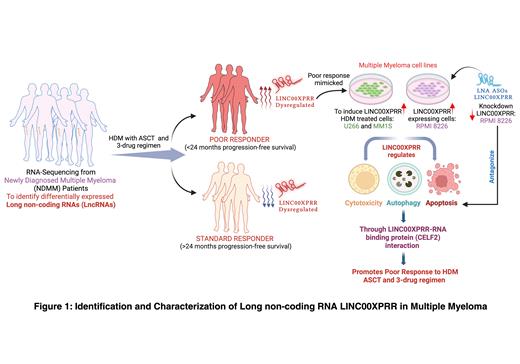
In order to address these issues, we performed transcriptome sequencing from NDMM patients which identified 244 differentially expressed lncRNAs in poor responders (indicated as <24 months progression free survival (PFS)) to three-drug regimen, HDM and ASCT (n=38) compared to standard responders (indicated as >24 months PFS) to three-drug regimen, HDM and ASCT (n=78). Our analysis revealed LINC00XPRR (lncRNA00Xpoor responder-related) as one of the top-most upregulated novel lncRNA associated with poor responders to standard myeloma care. We next validated LINC00XPRR high expression in single cell RNA-sequencing data in NDMM patient samples. Multiplexed fluorescent RNA in situ hybridization (mFISH) analysisshowed that LINC00XPRR expression increased in melphalan treated myeloma cell lines and is highly expressed in bone marrow aspirates from patient samples. We further determined higher levels of LINC00XPRR in the cytoplasmic compartment of the cells compared to nuclear localization through QuPath analysis. LINC00XPRR mechanistically and epigenetically modulates cytotoxic and apoptotic phenotypes in myeloma cell lines when overexpressed in U266B1 and MM1.S cells or using RPMI8226 CRISPR/Cas9 knockdown system and in appropriate multiple myeloma-associated mouse models. Additionally, we used RNA immunoprecipitation (RIP) combined with qPCR to determined that LINC00XPRR directly interacts with CELF2, RNA-binding protein. Lastly, using target specific LINC00XPRR designed locked-nucleic acid antisense oligonucleotides (LNA ASOs) to knockdown LINC00XPRR, we saw an increase in apoptotic phenotypes indicating clinical potential of LNA ASOs-mediated LINC00XPRR knockdown.
Overall, we are the first to identify lncRNAs associated with poor response in NDMM patients to standard three-drug induction chemotherapy followed by HDM and ASCT and show that LINC00XPRR enhanced expression may be both an important biomarker and target for future therapeutic interventions. Our current studies focus on the biology of lncRNAs in myeloma and the mechanism by which increased expression of LINC00XPRR is associated with poor outcomes in patients with NDMM receiving standard induction therapy, HDM and ASCT.
Topics: multiple myeloma, long non-coding RNAs, LNA ASOs, RNA therapeutics, treatment response, RNA sequencing, apoptosis
Disclosures
Schroeder:Novo nordisk: Consultancy; Incyte: Honoraria; GSK: Consultancy; Kura: Membership on an entity's Board of Directors or advisory committees; Sorrento therapeutics: Membership on an entity's Board of Directors or advisory committees; Marker Therapeutics: Membership on an entity's Board of Directors or advisory committees. Vij:Pfizer: Honoraria; Karyopharm: Honoraria; Harpoon: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Janssen: Honoraria; Legend: Honoraria; Takeda: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding. DiPersio:Magenta: Current holder of stock options in a privately-held company, Other: Ownership Investment, Patents & Royalties; WUGEN: Current holder of stock options in a privately-held company, Other: Ownership Investment, Patents & Royalties, Research Funding; Vertex: Consultancy; Macrogenics: Research Funding; Bioline: Consultancy; Rivervest: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal